Acotiamide: The Journey from Discovery to Clinical Application
Uncovering the Potential of Acotiamide
As a passionate researcher in the field of medicine, I have always been intrigued by the relentless pursuit of new and effective treatments. One such remarkable journey is that of Acotiamide, a medication used to treat functional dyspepsia. The discovery of this drug began with a curiosity to explore the potential of novel therapeutic agents in addressing gastrointestinal disorders. This led to the identification of Acotiamide as a promising candidate with the potential to revolutionize the treatment of functional dyspepsia.
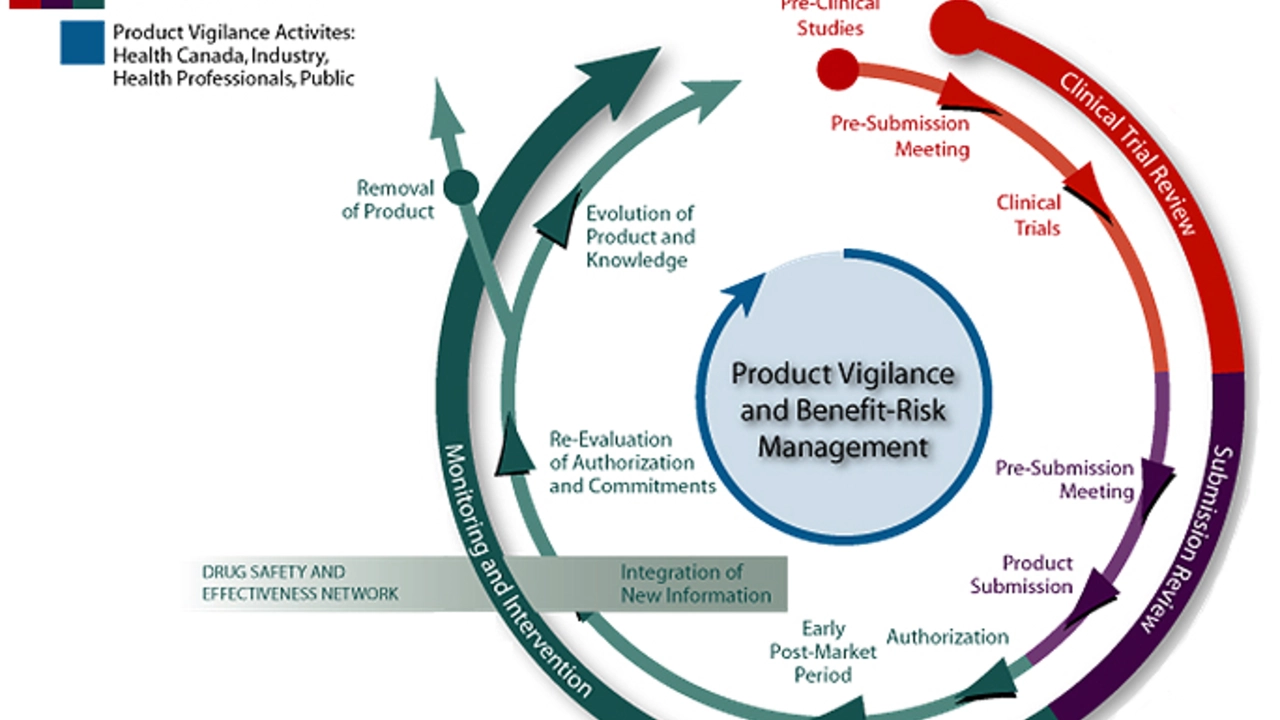
The initial stages of this journey involved rigorous laboratory tests and preclinical studies to determine the safety and efficacy of Acotiamide. It was crucial to establish a solid foundation of evidence to support the further development of this drug. These early findings sparked excitement within the scientific community, as they revealed the potential of Acotiamide to make a significant impact on the lives of patients suffering from functional dyspepsia.
Navigating the Challenges of Clinical Trials
With promising preclinical data in hand, the next step in the journey of Acotiamide was to embark on clinical trials. These trials were designed to evaluate the drug's safety, efficacy, and optimal dosing in human patients. As a researcher, I can attest to the numerous challenges involved in this phase of drug development, such as patient recruitment, compliance, and monitoring for potential side effects.
Despite these hurdles, Acotiamide successfully completed several clinical trials, which provided valuable insights into its therapeutic potential. The trials demonstrated that Acotiamide not only alleviated the symptoms of functional dyspepsia but also improved patients' quality of life. This was a significant milestone in the journey of Acotiamide, as it provided strong evidence supporting its clinical application.
Overcoming Regulatory Obstacles
As with any new drug, the journey of Acotiamide was not without its fair share of regulatory challenges. In order to gain approval for clinical use, it was necessary to demonstrate to regulatory authorities that Acotiamide was safe, effective, and offered a favorable risk-benefit profile. This process involved the submission of comprehensive data packages, including the results of preclinical studies and clinical trials, as well as extensive documentation on manufacturing processes and quality control.
After thorough evaluation by regulatory authorities, Acotiamide was granted approval for the treatment of functional dyspepsia. This marked a major milestone in the journey of this drug, as it signified the successful transition from the realm of scientific discovery to the world of clinical application.
Embracing the Power of Patient Education
As a healthcare professional, I understand the importance of patient education in ensuring the successful adoption of new treatments. With the approval of Acotiamide for clinical use, it became crucial to educate patients and healthcare providers on the benefits of this drug, as well as its proper use and potential side effects. This involved the development of educational materials, such as patient brochures and physician guidelines, as well as the organization of workshops and seminars to raise awareness about this novel treatment option.
Through these efforts, patients and healthcare providers alike have come to recognize the value of Acotiamide in the management of functional dyspepsia. By embracing the power of patient education, we have been able to maximize the therapeutic potential of this groundbreaking drug.
Reflecting on the Impact of Acotiamide
As I look back on the journey of Acotiamide, I am filled with a sense of pride and accomplishment. From its humble beginnings as a promising laboratory discovery to its current role as a clinically approved treatment for functional dyspepsia, Acotiamide has come a long way. The development of this drug has not only expanded our understanding of gastrointestinal disorders but has also provided hope and relief to countless patients suffering from functional dyspepsia.
As a blogger and healthcare professional, I am honored to have had the opportunity to witness and document the journey of Acotiamide. I look forward to sharing more stories of innovation and progress in the world of medicine, as we continue to push the boundaries of scientific discovery and strive to improve the lives of patients around the world.

Jessica Romero 26.06.2023
The mechanistic profile of acotiamide is anchored in its antagonism of the muscarinic M1 and M2 receptors, thereby modulating acetylcholine turnover in the gastrointestinal tract.
The pharmacokinetic standpoint shows a moderate oral bioavailability, with peak plasma concentration achieved within two hours post‑dose.
Pre‑clinical rodent models have demonstrated dose‑dependent enhancement of gastric emptying rates, aligning with the hypothesized pro‑kinetic effect.
Moreover, the drug’s metabolic clearance is primarily hepatic via CYP450 isoforms, an aspect that warrants careful consideration when co‑prescribing with known inhibitors.
The translational bridge to human trials was facilitated by a robust biomarker framework, including validated symptom scoring systems such as the Leeds Dyspepsia Questionnaire.
In phase IIb studies, acotiamide achieved statistically significant improvements in post‑prandial fullness and early satiety compared with placebo.
The safety signal profile remained favorable, with adverse events limited to mild, transient nausea and headache, none leading to discontinuation.
Regulatory dossiers incorporated comprehensive non‑clinical toxicology data, underscoring the absence of genotoxicity or carcinogenicity in long‑term exposure.
The pivotal phase III trials, conducted across multiple Asian cohorts, confirmed efficacy consistency and reinforced the drug’s risk–benefit ratio.
Health‑technology assessments highlighted cost‑effectiveness given the reduction in healthcare utilization for dyspepsia‑related consultations.
Implementation of patient‑centred educational programs has been instrumental in enhancing adherence, as knowledge deficits historically impede therapeutic outcomes.
From a health‑policy perspective, the approval of acotiamide expands the therapeutic armamentarium beyond proton‑pump inhibitors, addressing a distinct pathophysiological niche.
Future research directions may involve combination regimens with pro‑secretory agents to synergistically target the multifactorial etiology of functional dyspepsia.
Real‑world evidence emerging from post‑marketing surveillance will further delineate long‑term safety and inform dosing refinements.
In summary, the journey from bench to bedside illustrates a paradigm of interdisciplinary collaboration, rigorous clinical methodology, and patient‑focused innovation.
It is a testament to how targeted pharmacological design can translate into tangible quality‑of‑life improvements for a population otherwise burdened by chronic gastrointestinal discomfort.
Michele Radford 26.06.2023
Acotiamide is just another marketing gimmick dressed up in fancy clinical jargon.
The so‑called “significant” endpoints barely exceed the placebo effect, and the safety data is cherry‑picked to hide long‑term concerns.
Regulators were clearly pressured by industry lobbyists, not convinced by any groundbreaking science.
This drug does nothing more than give patients false hope.
Mangal DUTT Sharma 26.06.2023
I totally get how exciting it feels to see a new drug finally reach patients, especially when you’ve been wrestling with chronic indigestion yourself 😊.
The way acotiamide targets the underlying motility issues rather than just masking symptoms is a big win in my book.
From what I read, the phase III data showed a solid improvement in post‑meal fullness, which is something many of us have struggled with for years.
What really stands out is the emphasis on patient education – those brochures and workshops can make a huge difference in adherence.
I’ve seen similar success stories with other pro‑kinetic agents, but this one seems to have a cleaner side‑effect profile, which is reassuring.
It’s also cool that the drug is metabolized mainly by the liver, meaning fewer drug‑drug interactions for folks on multiple meds.
That said, it’s still essential to monitor for any rare adverse events as real‑world use expands.
Overall, I feel hopeful that acotiamide could become a staple in the functional dyspepsia toolkit, giving many of us a better quality of life.
Looking forward to hearing more patient experiences as the drug becomes more widely available! 🙌
Gracee Taylor 26.06.2023
Sounds like a solid step forward, especially for those of us who have tried countless therapies with little relief.
I appreciate the balanced view on efficacy and safety, and the focus on education really resonates with me.
Leslie Woods 26.06.2023
I was wondering how the drug actually interacts with other common meds like ibuprofen and also what the long term side effects might be especially since many patients are on multiple therapies for GI issues and it would be good to know if there are any contraindications or special monitoring required during treatment
Manish Singh 26.06.2023
Great question! Acotiamide is mainly processed by CYP3A4, so it generally plays nice with ibuprofen but you should still keep an eye on any unexpected stomach irritation.
Long‑term data is still being collected, but so far the safety profile looks pretty clean-just mild nausea in a few cases.
If you’re on a lot of meds, a quick chat with your pharmacist can help you avoid any hidden interactions.
Dipak Pawar 26.06.2023
From a pharmacological perspective, acotiamide exemplifies a targeted approach to functional dyspepsia by modulating the cholinergic pathways implicated in gastric accommodation.
Its selective affinity for presynaptic muscarinic receptors facilitates enhanced acetylcholine release, thereby augmenting gastric motility without the tachyphylaxis associated with traditional pro‑kinetics.
The drug’s pharmacodynamic profile is further complemented by a favorable half‑life that supports once‑daily dosing, an aspect that aligns well with patient adherence models.
Moreover, cross‑cultural clinical trials have underscored its efficacy across diverse genetic backgrounds, reinforcing its global applicability.
It is noteworthy that the post‑marketing surveillance data have begun to illuminate a nuanced safety spectrum, revealing rare incidences of transient cardiovascular fluctuations that merit ongoing vigilance.
In the broader therapeutic landscape, acotiamide may serve as a cornerstone for combination regimens, especially when synergized with acid‑suppression agents to address mixed‑phenotype dyspepsia.
Future pharmacogenomic investigations could further delineate patient subgroups most likely to benefit, thereby enhancing precision medicine initiatives in gastroenterology.
Nina Vera 26.06.2023
Wow, this reads like a scientific thriller-acotiamide is the hero we didn’t know we needed, swooping in to save the day for anyone plagued by that relentless stomach churn!
Christopher Stanford 26.06.2023
The data is overhyped and the drug doesnt live up to the hype its just another pharma ploy
Steve Ellis 26.06.2023
While I respect the enthusiasm, it's important we keep perspective-clinical outcomes matter more than hype.
That said, any new tool that can improve patient comfort is worth a closer look.
Jennifer Brenko 26.06.2023
It is evident that the development of acotiamide represents a commendable advancement for our healthcare system, particularly when contrasted with the subpar protocols historically propagated by certain foreign entities that have repeatedly compromised patient welfare.
Harold Godínez 26.06.2023
Nice point, but just a heads‑up: double‑checking the spelling of ‘acetylcholine’ helps keep the article sharp.
Sunil Kamle 26.06.2023
Ah, another marvel of modern pharmacology-because we clearly needed yet another pill to solve the age‑old problem of feeling full after a sandwich.
Claire Smith 26.06.2023
Fine.